
December 2020 – By Sandy Yanez
Cannabinoids are a diverse set of chemical compounds that bind to special receptors in the human body which make up what is known as The Endocannabinoid System (ECS). The “key and lock” metaphor is often used to describe this process. The human body possesses specific binding sites (“locks”) on the surface of many cell types, and our body produces several endocannabinoids (“keys”) that bind to these cannabinoid receptors (CB) to activate or “unlock” them.
In 1992, researchers detected an endogenous substance that binds to cannabinoid receptors for the first time. This substance, known as Anandamide (AEA), comes from the Sanskrit word “Ananda” for bliss and amide, due to its chemical structure. A second endocannabinoid was discovered in 1995, 2-arachidonoylglycerol (2-AG). So far, these two endocannabinoids have been studied the most. Today, it is thought that about 200+ related substances exist, which resemble the endocannabinoids and complement their function in what has been termed the “entourage effect.” Several endocannabinoids not only bind to cannabinoid receptors, but also to a possible CB3 receptor (the GPR55 receptor), to vanilloid receptors and further receptors.
In addition to endocannabinoids, scientists have now identified cannabinoids found in the cannabis plant (phytocannabinoids) that work to mimic or counteract the effects of some endocannabinoids. Phytocannabinoids and terpenes are manufactured in resin glands (trichomes) present on the flowers and main fan leaves of late-stage cannabis plants. The amount of resin produced, and its cannabinoid content varies by plant gender, growing conditions and harvesting time. The chemical stability of cannabinoids in harvested plant material is affected by moisture, temperature, light and storage, but will degrade over time in any storage conditions.
When a cannabinoid causes a receptor to act in the same way as it would to a naturally occurring hormone or neurotransmitter, then it is labeled “agonist.” On the other hand, if the cannabinoid prevents the receptor from binding to the naturally occurring compound, thereby causing the resulting event (e.g., pain, appetite, alertness) to be altered or diminished, it is labeled “antagonist.” Research is mounting to better understand how specific cannabinoids can unlock (or lock in some cases) specific receptors.
Over 100 phytocannabinoids have been identified in the cannabis plant, many of which have documented medicinal value. Most are closely related or differ by only a single chemical part. The most talked-about and researched cannabinoids found in the cannabis plant are tetrahydrocannabinol (THC) for its psychoactive properties (“high feeling”) and cannabidiol (CBD) for its healing properties.
Cannabinoids can be administered by smoking, vaporizing, oral ingestion, transdermal patch, sublingual absorption, or rectal suppository.

An Endogenous Cannabinoid System (ECS), commonly referred to as an “Endocannabinoid System,” is found in every animal and regulates a broad range of biological functions. The ECS is a biochemical control system of neuromodulator lipids (molecules that include fats, waxes, sterols and fat-soluble vitamins such as vitamins A, D, E and K and others) and specialized receptors configured to accept certain cannabinoids. In general, a given receptor will accept only particular classes of compounds and will be unaffected by other compounds, just as a specific key is needed to open a lock.
Specialized receptors are located throughout the human body, including but not limited to, in the hippocampus (memory, learning), the cerebral cortex (decision-making, emotional behavior), the cerebellum (motor control, coordination), putamen (movement, learning), the hypothalamus (appetite, body temperature) and the amygdala (emotions). When a specific cannabinoid or combination of cannabinoids bind to a specialized receptor, an event or a series of events, is triggered in the cell, resulting in a change in the cell’s activity, its gene regulation and/or the signals that it sends to neighboring cells. This process is called “signal transduction.”
Clinical endocannabinoid deficiency (CECD) is a proposed spectrum disorder that has been implicated in a range of illnesses, including fibromyalgia, migraine, and irritable bowel syndrome. So far, little clinical research has been conducted on this speculative disorder. It is quite possible that these very common conditions may respond favorably to cannabinoid therapies. However, this will only happen if more research is conducted.
The primary cannabinoid receptors are identified as Cannabinoid type 1 receptors (CB1-R) and Cannabinoid type 2 receptors (CB2-R). The receptors can be “unlocked” by three kinds of cannabinoids:
Endocannabinoids – endogenous-fatty-acid cannabinoids produced naturally in the body (e.g., anandamide and 2-AG)
Phytocannabinoids – concentrated in the oily resin of the buds and leaves of plants such as cannabis (e.g., THC and CBD)
Synthetic cannabinoids – manufactured by artificial means such as in a laboratory (e.g., Dronabinol and Sativex)
First detected in the brain, science now shows that CB1-R are also located in many other organs, connective tissues, gonads, and glands. CB1-R are not found in the medulla oblongata (the part of the brain stem responsible for respiratory and cardiovascular functions). CB1-R play an important role in the coordination of movements, spatial orientation, sensory perceptions (taste, touch, smell, hearing), cognitive performance and motivation.
The most important function of the CB1-R is the reduction of excessive or inadequate signaling by the neurotransmitters (messengers) in the brain. By the activation of the CB1-R, the hyperactivity or hypoactivity of the messengers (e.g., serotonin, dopamine) is regulated back into balance. For example, when THC binds to CB1-R, activity in the pain circuits is inhibited, thus resulting in reduced pain. Many other symptoms such as nausea, muscle spasticity and seizures can be alleviated or diminished with cannabinoid therapy.
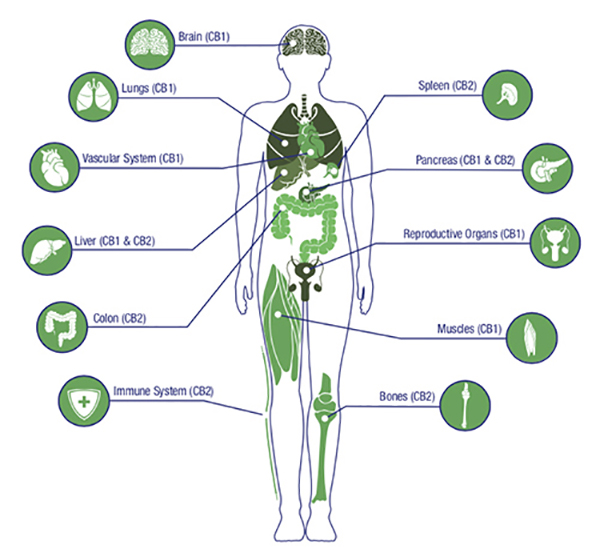
CB2-R are primarily associated with the immune system and found outside of the brain in such places as the gut, spleen, liver, heart, kidneys, bones, blood vessels, lymph cells, endocrine glands, and reproductive organs. For example, CBD is keyed to CB2-R, and good evidence shows CBD is a beneficial therapeutic strategy to lessen the impact of inflammatory and neuro-inflammatory diseases.
Until recently, it was believed that CB-2R played no role with nerve cells or bundles. However, studies now show that it also plays an important role in the signal processing of the brain.
A third receptor that gets little attention is the transient receptor potential vanilloid-type one (TRPV1). The function of TRPV1 is to detect and regulate body temperature. In addition, TRPV1 is responsible for the sensations of extreme external heat and pain and is subject to desensitization. Therefore, if continuously stimulated, the pathway will eventually slow down or even stop. This raises therapeutic possibilities for agents to effectively treat certain kinds of neuropathic pain.
Since the initial discovery of the CB1 receptor site by Allyn Howlett and William Devane in 1988, it has been an “accepted” fact that CBD, unlike THC, has little binding affinity for the CB1 receptor. Unfortunately, this assumption was not based on science. New data emerging from the international cannabinoid research community indicates that CBD interacts directly with the CB1 receptor site in ways that are therapeutically relevant. CBD parks at a different docking site on the CB1-R that is functionally distinct from THC’s orthosteric binding site. CBD attaches to what is known as an “allosteric” binding site on CB1-R. When CBD docks at the receptor, it does not initiate a signaling cascade. It does, however, influence how the receptor responds to stimulation by THC and the endogenous cannabinoids. Allosteric modulation of CB1-R changes the conformation (shape) of the receptor, and this can have a dramatic impact on the efficiency of cell signaling.
A positive allosteric modulator that enhances CB1 receptor signaling indicates that CBD could be helpful treating diseases linked to endocannabinoid deficits (such as anorexia, migraines, irritable bowel, fibromyalgia, and PTSD), in addition to treating conditions associated with endocannabinoid excess or overactivity (e.g., obesity, metabolic disorders, liver disease, cardiovascular issues).
The concept of the entourage effect was introduced in 1998 by Israeli scientists Shimon Ben-Shabat and Raphael Mechoulam. The theory is that cannabinoids within the cannabis plant work together through a network of coincidental relationships as part of a greater organism and affect the body in a mechanism similar to the body’s own endocannabinoid system. Basically, these compounds work better together than in isolation.
The longstanding, successful use of cannabis as a whole makes it necessary to find a rationale for its medicinal superiority in comparison to products containing isolated, single components of the cannabis plant, or synthetic cannabinoids trying to replicate the natural components.
Research on the benefits of THC and CBD in isolation is well established. THC demonstrates analgesic, anti-emetic, and anti-inflammatory properties. CBD possesses anti-psychotic, anti-seizure, and anti-anxiety properties. However, evidence is mounting that by isolating these cannabinoids or creating them in a lab, that the resulting effects may have limited therapeutic use. It is also the reason visits to a doctor or emergency room have increased. When delivered in high concentrations, THC can cause overdosing. Although an acute THC overdose rarely requires medical intervention, the side effects can be very unpleasant. Good evidence now shows that THC and CBD work together. CBD is known to lock out THC at the CB1-R. Therefore, applying the entourage effect, increasing CBD in the case of an overdose may lessen the effects of THC.
Each cannabinoid offers unique medical properties. Many of them have been observed in clinical settings. Here are a few:
Cannabidiol (CBD)
CBD is the principal, non-psychoactive cannabinoid present in the cannabis plant that occurs second in concentration to THC. CBD itself has a long list of medical properties, primarily relieving medical conditions such as chronic pain, severe inflammation, migraines and headaches, arthritis, muscle spasms, epileptic seizures, mood swings associated with schizophrenia, and has also been shown to have anti-cancer properties. CBD has tremendous medical potential and is particularly true when the correct ratio of CBD to THC is applied to treat a particular condition. CBD acts as an antagonist at both the CB1 and CB2 receptors, yet it has a low binding affinity for both. This suggests that CBD’s mechanism of action is mediated by other receptors in the brain and body.
Tetrahydrocannabinol (THC)
Delta-9-tetrahydrocannabinol (THC) is a phytocannabinoid, and typically the most abundant cannabinoid present in cannabis products on the market today. THC can be derived from THCA by non-enzymatic decarboxylation during storage and consumption. It is responsible for the well-documented psychoactive effects experienced when consuming cannabis. THC mimics anandamide, your “bliss” molecule, to boost your mood by modulating the activity of dopamine, which gives you a sense of euphoria or “high.” Besides elevating moods, THC has similar benefits to THCA. Due to its cerebral effects, both elating and clarifying, THC can be used to help with focus and sleep issues. When you smoke or ingest cannabis, THC travels into the bloodstream and eventually binds to cannabinoid receptors throughout your body. These receptor sites affect memory, concentration, pleasure, coordination, sensory and time perception, appetite and many more important functions. Mild side effects of larger doses of THC can include anxiety, burning eyes, dry mouth, jitters, increased heart rate and/or shortness of breath (or at least the perception of such) and short-term memory loss.
Cannabidivarin (CBDV)
CBDV differs from CBD only by the substitution of a pentyl (5 carbon) for a propyl (3 carbon) sidechain. Although research on CBDV is still in its initial stages, recent studies have shown promise for its use in the management of epilepsy. This is due to its action at TRPV1 receptors and modulation of gene expression. CBDV research has also shown effective in the treatment of a variety of symptoms and conditions including seizures, Crohn’s disease, HIV/AIDS, Autism Spectrum disorder (ASD), and multiple sclerosis.
Cannabidiolic Acid (CBDA)
CBDA is the main form in which CBD exists in the cannabis plant, along with THCA (THC-acid). CBD is obtained through non-enzymatic decarboxylation from the acidic form of the cannabinoid, this reaction taking place when the compounds are heated. Heating or catalyzing CBDA transforms it into CBD, thereby increasing the total CBD level. Research shows higher concentrations of CBDA displayed more pronounced antimicrobial activity than CBD alone.
Unlike other cannabinoids, CBDA does not interact with the endocannabinoid system (ECS), neither does it bind directly with the CB1 or CB2 receptors. However, it may interact with COX-2, which is an enzyme associated with inflammation. CBDA might also influence serotonin, a transmitter associated with happiness and overall well-being. CBDA is therefore thought to deliver its therapeutic power to the body by interacting with enzymes and receptors rather than the ECS. It has been shown to CBDA affected serotonin levels which effects functions eating, motor skills, emotions, and digestion. CBDA interacts with the 5-HT receptors making this cannabinoid a powerful anticonvulsant.
Cannabigerol (CBG)
A non-psychoactive cannabinoid, CBG’s antibacterial effects can alter the overall effects of cannabis. CBG is known to kill or slow bacterial growth, reduce inflammation, (particularly in its acidic CBGA form,) inhibit cell growth in tumor/cancer cells, and promote bone growth. It is believed that CBG May also be able to help manage glaucoma due to its ability to reduce Inter ocular pressure. It acts as a low-affinity antagonist at the CB1 receptor. CBG pharmacological activity at the CB2 receptor is currently unknown.
CBG Has been found to inhibit the uptake of GABA, which causes a feeling of relaxation, a decreased in anxiety and a decrease in muscle tension.
CBG has also shown promise when it comes to fighting cancer. According to scientists, the compound has demonstrated that it can block the receptors that promote cancer-causing cells’ growth. According to researchers, CBG has shown the potential to serve as an active antibacterial agent, specifically MRSA.
One study was conducted to see how four cannabinoids – CBDV, CBD, THCV, and CBG – affect bladder contractions. By the end of the study, the researchers found that cannabigerol was the most effective in inhibiting muscle contractions in the bladder and concluded that it might prove beneficial for treating the different, uncomfortable symptoms related to bladder disorders.

Cannabinol (CBN)
CBN is a very mildly psychoactive cannabinoid that is produced from the degradation of THC. There is usually very little to no CBN in a fresh plant. CBN acts as a weak agonist at both the CB1 and CB2 receptors, with greater affinity for CB2 receptors than CB1. The degradation of THC into CBN is often described as creating a sedative effect. Studies have shown that CBN is a powerful anti-bacterial, anti-inflammatory, and neuroprotective agent (and is use as a treatment of ALS) to name just a few.
Cannabichromene (CBC)
Cannabichromene (CBC) is one of the most prevalent compounds in cannabis and hemp, yet it’s virtually unknown to many people. CBC is not psychoactive, so you can enjoy its benefits without experiencing the ‘high’ sensation commonly associated with cannabis. Evidence has shown that it may play a role in the anti-inflammatory and anti-viral effects of cannabis and may contribute to the overall analgesic effects of medical cannabis. A study done in March 2010 showed that CBC along with cannabidiol (CBD) and tetrahydrocannabinol (THC) have antidepressant effects. Another study showed that CBC helps promote neurogenesis.
CBC is an effective treatment for migraines and headaches. A 2013 study concluded that CBD might have the potential to promote neurogenesis, the growth and development of new brain tissue. This discovery could lead to new advancements in treating brain injuries and degenerative diseases like Alzheimer’s. CBC is revealing itself as an effective treatment for symptoms related to conditions like Inflammatory Bowel Disease (IBD), Crohn’s Disease, neuropathy and chronic pain.
Tetrahydrocannabivarin (THCV)
THCV is a minor cannabinoid found in only some strains of cannabis. The only structural difference between THCV and THC is the presence of a propyl (3 carbon) group, rather than a pentyl (5 carbon) group, on the molecule. Though this variation may seem subtle, it causes THCV to produce very different effects than THC. These effects include a reduction in panic attacks including those associated with PTSD without suppressing your emotions. THCV helps with appetite suppression and good news for persons suffering from Type 2 diabetes. Studies have shown that this cannabinoid can improve glucose tolerance and help regulate insulin levels to stabilize blood sugar levels.
Research has shown THCV slows or prevents some forms of bone degeneration and may actually promote the growth of new bone cells.
THCV is an analgesic and anti-inflammatory. A neuroprotectant, which means it protects the nervous system, it combined with both CB1 and CB2 receptors and can reduce muscular tremors associated with Parkinson’s disease, Alzheimer’s disease, and ALS.
Tetrahydrocannabinolic Acid (THCA)
THCA is the main constituent in raw cannabis. THCA converts to Δ9-THC when burned, vaporized, or heated at a certain temperature. THCA, CBDA, CBGA, and other acidic cannabinoids hold the most COX-1 and COX-2 inhibition, contributing to cannabis’ anti-inflammatory effects.
The neuroprotective qualities could benefit persons with neurodegenerative disorders like Alzheimer’s and Parkinson’s disease as well as those with dementia. This cannabinoid also acts as an antiproliferative noted in studies of prostate cancer. Other medicinal avenues supported include insomnia, muscle spasms, and pain.
A study observed that THC a holds a strong anti-inflammatory properties than CBD for the possible treatment of IBD such as Crohn’s disease and ulcerative colitis. THCA demonstrates potential for treating Huntington’s disease, and other neurodegenerative disorders.
References:
Alger B. E. (2013). Getting high on the endocannabinoid system. Cerebrum : the Dana forum on brain science, 2013, 14. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC3997295/
Appendino, G., Gibbons, S., Giana, A., Pagani, A., Grassi, G., Stavri, M., Smith, E., & Rahman, M. M. (2008). Antibacterial cannabinoids from Cannabis sativa: a structure-activity study. Journal of natural products, 71(8), 1427–1430. https://doi.org/10.1021/np8002673
Borrelli, F., Pagano, E., Romano, B., Panzera, S., Maiello, F., Coppola, D., De Petrocellis, L., Buono, L., Orlando, P., & Izzo, A. A. (2014). Colon carcinogenesis is inhibited by the TRPM8 antagonist cannabigerol, a Cannabis-derived non-psychotropic cannabinoid. Carcinogenesis, 35(12), 2787–2797. https://doi.org/10.1093/carcin/bgu205
Chye, Y., Christensen, E., Solowij, N., &Yucel, M. The Endocannabinoid System and Cannabidiol’s Promise for the Treatment of Substance Use Disorder. Front Psychiatry. 2019;10-63. https://www.frontiersin.org/articles/10.3389/fpsyt.2019.00063/full
DeLong, G. T., Wolf, C. E., Poklis, A., & Lichtman, A. H. (2010). Pharmacological evaluation of the natural constituent of Cannabis sativa, cannabichromene and its modulation by Δ(9)-tetrahydrocannabinol. Drug and alcohol dependence, 112(1-2), 126–133. https://doi.org/10.1016/j.drugalcdep.2010.05.019
De Petrocellis, L., Orlando, P., Moriello, A. S., Aviello, G., Stott, C., Izzo, A. A., & Di Marzo, V. (2012). Cannabinoid actions at TRPV channels: effects on TRPV3 and TRPV4 and their potential relevance to gastrointestinal inflammation. Acta physiologica (Oxford, England), 204(2), 255–266. https://doi.org/10.1111/j.1748-1716.2011.02338.x
Ferber, S. G., Namdar, D., Hen-Shoval, D., Eger, G., Koltai, H., Shoval, G., Shbiro, L., & Weller, A. (2020). The “Entourage Effect”: Terpenes Coupled with Cannabinoids for the Treatment of Mood Disorders and Anxiety Disorders. Current neuropharmacology, 18(2), 87–96. https://doi.org/10.2174/1570159X17666190903103923
Hill, A. J., Mercier, M. S., Hill, T. D., Glyn, S. E., Jones, N. A., Yamasaki, Y., Futamura, T., Duncan, M., Stott, C. G., Stephens, G. J., Williams, C. M., & Whalley, B. J. (2012). Cannabidivarin is anticonvulsant in mouse and rat. British journal of pharmacology, 167(8), 1629–1642. https://doi.org/10.1111/j.1476-5381.2012.02207.x
Idris, A. I., & Ralston, S. H. (2012). Role of cannabinoids in the regulation of bone remodeling. Frontiers in endocrinology, 3, 136. https://doi.org/10.3389/fendo.2012.00136
Jadoon, K. A., Ratcliffe, S. H., Barrett, D. A., Thomas, E. L., Stott, C., Bell, J. D., O’Sullivan, S. E., & Tan, G. D. (2016). Efficacy and Safety of Cannabidiol and Tetrahydrocannabivarin on Glycemic and Lipid Parameters in Patients With Type 2 Diabetes: A Randomized, Double-Blind, Placebo-Controlled, Parallel Group Pilot Study. Diabetes care, 39(10), 1777–1786. https://doi.org/10.2337/dc16-0650
Maroon, J., & Bost, J. (2018). Review of the neurological benefits of phytocannabinoids. Surgical neurology international, 9, 91. https://doi.org/10.4103/sni.sni_45_18
Nadal, X., Del Río, C., Casano, S., Palomares, B., Ferreiro-Vera, C., Navarrete, C., Sánchez-Carnerero, C., Cantarero, I., Bellido, M. L., Meyer, S., Morello, G., Appendino, G., & Muñoz, E. (2017). Tetrahydrocannabinolic acid is a potent PPARγ agonist with neuroprotective activity. British journal of pharmacology, 174(23), 4263–4276. https://doi.org/10.1111/bph.14019
Nadolska, K., & Goś, R. (2008). Mozliwości zastosowania kannabinoidów w leczeniu jaskry [Possibilities of applying cannabinoids’ in the treatment of glaucoma]. Klinika oczna, 110(7-9), 314–317.
Nallathambi, R., Mazuz, M., Ion, A., Selvaraj, G., Weininger, S., Fridlender, M., Nasser, A., Sagee, O., Kumari, P., Nemichenizer, D., Mendelovitz, M., Firstein, N., Hanin, O., Konikoff, F., Kapulnik, Y., Naftali, T., & Koltai, H. (2017). Anti-Inflammatory Activity in Colon Models Is Derived from Δ9-Tetrahydrocannabinolic Acid That Interacts with Additional Compounds in Cannabis Extracts. Cannabis and cannabinoid research, 2(1), 167–182. https://doi.org/10.1089/can.2017.0027
National Center for Biotechnology Information (2020). PubChem Compound Summary for CID 5281969, Anandamide. Retrieved December 4, 2020 from https://pubchem.ncbi.nlm.nih.gov/compound/Anandamide.
Noriko Shinjyo, Vincenzo Di Marzo, The effect of cannabichromene on adult neural stem/progenitor cells, Neurochemistry International,Volume 63, Issue 5, (2013),432-437,ISSN 0197-0186, https://doi.org/10.1016/j.neuint.2013.08.002
Oz, M., Jaligam, V., Galadari, S., Petroianu, G., Shuba, Y. M., & Shippenberg, T. S. (2010). The endogenous cannabinoid, anandamide, inhibits dopamine transporter function by a receptor-independent mechanism. Journal of neurochemistry, 112(6), 1454–1464. https://doi.org/10.1111/j.1471-4159.2009.06557.x
Pacher, P., Bátkai, S., & Kunos, G. (2006). The endocannabinoid system as an emerging target of pharmacotherapy. Pharmacological reviews, 58(3), 389–462. https://doi.org/10.1124/pr.58.3.2
Pagano, E., Montanaro, V., Di Girolamo, A., Pistone, A., Altieri, V., Zjawiony, J. K., Izzo, A. A., & Capasso, R. (2015). Effect of Non-psychotropic Plant-derived Cannabinoids on Bladder Contractility: Focus on Cannabigerol. Natural product communications, 10(6), 1009–1012.
Reggio P. H. (2010). Endocannabinoid binding to the cannabinoid receptors: what is known and what remains unknown. Current medicinal chemistry, 17(14), 1468–1486. https://doi.org/10.2174/092986710790980005
Russo E. B. (2018). Cannabis Therapeutics and the Future of Neurology. Frontiers in integrative neuroscience, 12, 51. https://doi.org/10.3389/fnint.2018.00051
Russo E. B. (2011). Taming THC: potential cannabis synergy and phytocannabinoid-terpenoid entourage effects. British journal of pharmacology, 163(7), 1344–1364. https://doi.org/10.1111/j.1476-5381.2011.01238.x
Wargent, E. T., Zaibi, M. S., Silvestri, C., Hislop, D. C., Stocker, C. J., Stott, C. G., Guy, G. W., Duncan, M., Di Marzo, V., & Cawthorne, M. A. (2013). The cannabinoid Δ(9)-tetrahydrocannabivarin (THCV) ameliorates insulin sensitivity in two mouse models of obesity. Nutrition & diabetes, 3(5), e68. https://doi.org/10.1038/nutd.2013.9
Weydt, P., Hong, S., Witting, A., Möller, T., Stella, N., & Kliot, M. (2005). Cannabinol delays symptom onset in SOD1 (G93A) transgenic mice without affecting survival. Amyotrophic lateral sclerosis and other motor neuron disorders : official publication of the World Federation of Neurology, Research Group on Motor Neuron Diseases, 6(3), 182–184. https://doi.org/10.1080/14660820510030149
Zamberletti, E., Gabaglio, M., Woolley-Roberts, M., Bingham, S., Rubino, T., & Parolaro, D. (2019). Cannabidivarin Treatment Ameliorates Autism-Like Behaviors and Restores Hippocampal Endocannabinoid System and Glia Alterations Induced by Prenatal Valproic Acid Exposure in Rats. Frontiers in cellular neuroscience, 13, 367. https://doi.org/10.3389/fncel.2019.00367